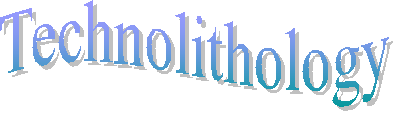Presence of improper aggregate – silicites, black clay shales (±tuffs,
±tuffites) with pyrite and clay minerals (Hawkins, Pinches [4]) and Ca(OH)2;
Formation of secondary sulphates ettringite Ca4Al2[(OH)12 / SO4] . 6 H2O, thaumasite Ca3H2[CO3 / SO4 / SiO4] . 13 H2O and gypsum Ca2(SO4)2 . 2 H2O (macroscopically visible due to white margins formatted round the black aggregate particles). Their formation closely relates to pyrite weathering processes, which can be found especially in black clay shales. Degradation of concrete drill cores relates also to growth pressure of rhombohedral calcite crystals in concrete matrix (Hartshorn, Sharp, Swamy [3]).
Thaumasite formation in experimental conditions studied (Crammond, Halliwell [2]). They verified thaumasite formation of neutral sulphates ions added to concrete or by sulphur acid action on concrete Oberholster, van Aardt, Brandt [7]).
It can not be excluded also affects of surrounding environment and the bedrock. The rocks containing sulphides and organic matters are altered by acid solutions by the formation of more stabile mineral forms. The typical example is pyrite decaying with the formation of limonite and sulphur acid. It can be demonstrated (Hobbs, Taylor 2000): 2FeS2 + 6H2O + 7O2 = 2Fe(OH)2 + 4H2SO4 . 4FeS2 + 15O2 + 8H2O = 2Fe2O3 + 8H2SO4 By the reaction of sulphur acid with surrounding CSH or CH (or with gels formatted by ASR) growth pressure of neogenic minerals breaks concrete. Limonitization of pyrite was verified in all samples.
ASR reaction (in a limited degree – it was verified in 3 samples)
Formation of secondary sulphates ettringite Ca4Al2[(OH)12 / SO4] . 6 H2O, thaumasite Ca3H2[CO3 / SO4 / SiO4] . 13 H2O and gypsum Ca2(SO4)2 . 2 H2O (macroscopically visible due to white margins formatted round the black aggregate particles). Their formation closely relates to pyrite weathering processes, which can be found especially in black clay shales. Degradation of concrete drill cores relates also to growth pressure of rhombohedral calcite crystals in concrete matrix (Hartshorn, Sharp, Swamy [3]).
Thaumasite formation in experimental conditions studied (Crammond, Halliwell [2]). They verified thaumasite formation of neutral sulphates ions added to concrete or by sulphur acid action on concrete Oberholster, van Aardt, Brandt [7]).
It can not be excluded also affects of surrounding environment and the bedrock. The rocks containing sulphides and organic matters are altered by acid solutions by the formation of more stabile mineral forms. The typical example is pyrite decaying with the formation of limonite and sulphur acid. It can be demonstrated (Hobbs, Taylor 2000): 2FeS2 + 6H2O + 7O2 = 2Fe(OH)2 + 4H2SO4 . 4FeS2 + 15O2 + 8H2O = 2Fe2O3 + 8H2SO4 By the reaction of sulphur acid with surrounding CSH or CH (or with gels formatted by ASR) growth pressure of neogenic minerals breaks concrete. Limonitization of pyrite was verified in all samples.
ASR reaction (in a limited degree – it was verified in 3 samples)
During the study of concrete samples was found by optical microscopy that even highly degraded concrete contents anhydrated clinker minerals. C3S and C2S are well distinguishable in some cases. Tetracalciumalumoferrite is the most marked in case of Portland clinker. Thaumasite, ettringite, gypsum and calcite were always identified within neogenic minerals. Rock association: pyrite bearing rock (clay shale, tuff, tuffite) and silica rocks were always identified in aggregate.
It was verified that in studied samples thaumasite often with ettringite were formed gradually of ASR gels. Their occurrence was confirmed by microanalyses. It can be formed hypothesis that important factor of rupture deformation of concrete matrix with formation of fine fissures are not only ASR gels but also following reactions induced by sulphur and hydrocarbon acids, hydroxides of Al and Ca together with ASR gels with the formation of especially thaumasite and in case of over-abundance of Ca2+ + Al3+ also ettringite. Gypsum needle-like crystals are formed in case of Ca ions presence (after formation of ettringite) together with low concentrated sulphur acid. Calcite is formed as a latest mineral in case of presence of remains of Ca hydroxide (Photo 1-10).
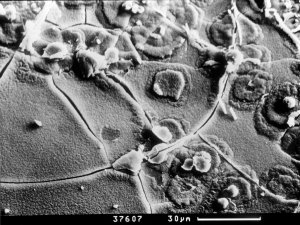 Figure 1: Formation of radial-like forms of portlandite of gel. SEM - CAM SCAN. Photo V. Vávra. |
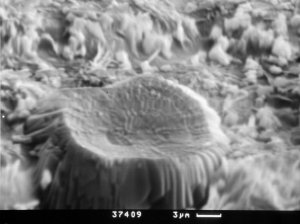 Figure 2: Detail view – one of formatting forms. SEM - CAM SCAN. Photo V. Vávra. |
 Figure 3: Formation of first thaumasite and ettringite crystals. SEM - CAM SCAN. Photo P. Sulovský. |
 Figure 4: Advanced state of thaumasite formation. SEM - CAM SCAN. Photo P. Sulovský. |
 Figure 5: Needle-like forms of thaumasite beside decaying gel. SEM - CAM SCAN. Photo P. Sulovský. |
 Figure 6: Ettringite, thaumasite, gel, portlandite. SEM - CAM SCAN. Photo V. Vávra. |
 Figure 7: Clumps of ettringite needles. SEM - CAM SCAN. Photo V. Vávra. |
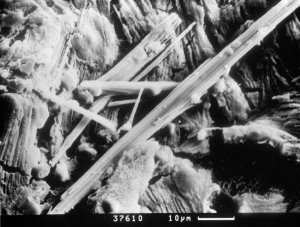 Figure 8: Columnar shape forms of thaumasite. SEM - CAM SCAN. Photo V. Vávra. |
 Figure 9: Rhombohedral calcite crystals on thaumasite in highway concrete. SEM - CAM SCAN. Photo V. Vávra. |
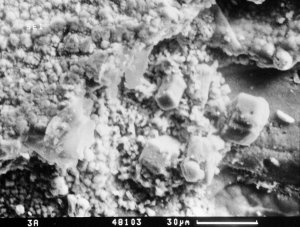 Figure 10: Rhombohedral calcite crystals beside thaumasite on the surface of interlocking concrete pavement. SEM - CAM SCAN. Photo P. Sulovský. |
Hobbs, Taylor [5] observed that in saturated concrete some or all gypsum crystals can react with hydrated calciumaluminate and form ettringite (usual sulphate corrosion). Thaumasite can be formed in relation with decreasing pH (below 12) when gypsum can react with CSH and calcite. Formation of thaumasite is more presumable in case of action of magnesium sulphate solution (solute in ground water). Thaumasite and ettringite formation is in close relation with expansion. This process forms fine fissures, which parallels the surface in subsurface zone of concrete.
It can be understood that ettringite is usual product of hydration and occurs both in fresh and degraded concretes. Ettringite causes concrete decaying only in case of its excessive formation, which increases with increasing age of concrete. Ettringite can not crystallize in free voids of concrete microstructure. Ettringite occurrence, lower than critical can only signalize alteration of microstructure (together with alteration of concrete properties) but need not lead to concrete decay.