Thaumasite formation affected by aggregate composition
in concrete in the
Miroslava Gregerová*)
Pavel Pospíšil**)
*)
**) Brno University of Technology,
Faculty of Civil Engineering, Dept. of Geotechnics, Veveri 95, 662 37 Brno,
Czech Republic, pospisil.p@fce.vutbr.cz
Abstract
Significance of optical study of cementitious materials
(matrix and filling material) becomes very interesting and important during
several last years. Study is focused not only on mineral composition of matrix
in concrete but especially on petrographic characteristic of aggregates.
Collection of 14 concrete samples was optically studied and petrographically
characterized. Aggregates of these samples can be macroscopically divided in
two groups, which mean that aggregates were formed in different geological
environments. Because phthanites (Paleozoic silica rock) and porcelanites were
observed in aggregates it had to be verified formation of ASR reaction
products.
Keywords
Thaumasite, concrete, degradation, petrographic
characteristic, aggregate
Introduction
Concrete degradation usually results of parallel action
of physical, chemical and biological processes, which can closely involved with
improper combination of aggregates and matrix, water and surrounding
conditions. Results of micropetrographic analyses of aggregates, EDX analyses
carried out on raster electron microscope CamScan supplemented with chemical
analyses of separated parts of highway concretes in various stages of
degradation are presented in this paper.
Petrographic characteristic
Micropetrographic aggregate analyses
of studied highway concretes proved differences in their rock composition as
shown in Fig. 1.
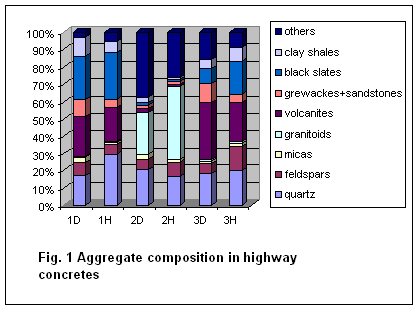
Basic differences were in amount of
black clay shales, granitoids and silicites in aggregate composition. It was
unambiguously proved that the lowest degraded concretes were with the highest
amount of granitoids. On the contrary the highest degraded were concretes with
prevailing portion of black clay shales and silicites in aggregate composition.
Percentage composition of aggregate in the highest degraded part of drill core
is shown in Fig. 2.
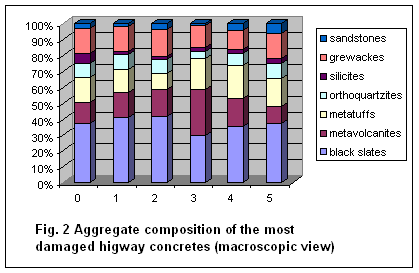
Concurrently with micropetrographic analyses of
highway concrete aggregates we focused on assessment of two different aggregate
deposits. The first of one is opened in old submarine volcanic rocks. The
second one is in granitoid rocks.
In comparison between drill cores 1, 3 and 7 are
samples 1 and 7 very similar in aggregate composition. Variability in aggregate
composition for concrete production probably reflects original rock heterogeneity
in the rock massif in the quarry.
The drill core No.: 2 is in
good quality, without significant cracks, without alteration in material
composition and without formation of secondary minerals. Granitoid rocks predominate
over metabasalts, shales and cemented detrital sediments. Studied rock
composition leads to assumption about ASR, which was not anticipated in
concretes in the
Aggregate alkali reaction
Presence of “active SiO2”
included in aggregate is considered as a basic condition for reactivity of
aggregate with alkali (Arya, Buenfeld, Newman [1]).
They are mostly opal, chalcedony, trydimite or crystobalite. These minerals
occur usually in rocks such as tuff, tuffite, volcanic glass, chert etc. These
rock types occur in concrete aggregate (not only of highway). Sometimes we can
find porcelain jasper (porcelanite), which is contact metamorphosed rock
(burned clays and marls) and was formed probably on the contact with basalt. It
is inhomogeneous in color, maculose with conchoidal fracture and usually
contains crystobalite, spurrite, larnite and other high-temperature minerals.
Besides the above mentioned rocks we
simultaneously verified occurrence of black clay shale or tuff and tuffite with
pyrite.
If we assume average chemical
composition of Portland clinker 21% SiO2, 5% Al2O3,
3% Fe2O3, 64% CaO, 3% MgO, 2,5% SO3 and 0,4%
of alkali oxides then will be formed approximately 0,8% CaO (free calcium
dioxide), 55,5% 3CaO.SiO2 (tricalciumsilicate), 17,8% 2CaO.SiO2
(dicalciumsilicate), 8,3% 3CaO.Al2O3
(tricalciumaluminate) and 8,3% 4CaO.Al2O3.Fe2O3
(tetracalciumalumoferrite).
The high content of
tetracalciumalumoferrite is in all concrete samples. Separate portlandite
tables and accumulations occur in micritic matrix. Voids of the highly degraded
concrete are along edges or whole volume filled by needle-like crystals of
ettringite (Ca6Al2O6(SO4)3.32H2O
or thaumasite Ca3 H2 [CO3/SO4/ SiO4]
.13 H2O and gypsum Ca2(SO4)2. 2 H2O.
Occurrence of these minerals as verified by X-ray and EDX analyses.
The gel coatings, which were ripped
by impact of electron beams occur along the edge of
some fissures – gradual water release. As we have confirmed by element
distribution maps amorphous gels have variable composition. Gradually developed
individual mineral phases formed of these gels were verified by many images.
Conclusions
Study of concrete samples verified
following degradation factors:
-
Presence of
improper aggregate – silicites, black clay shales (±tuffs, ±tuffites) with
pyrite and clay minerals (Hawkins, Pinches [4]) and Ca(OH)2;
-
Formation of
secondary sulphates ettringite Ca4Al2[
(OH)12/ SO4]. 6 H2O, thaumasite Ca3
H2 [CO3/SO4/ SiO4] .13 H2O
and gypsum Ca2(SO4)2.
2 H2O (macroscopically visible due to white margins formatted round
the black aggregate particles). Their formation closely relates to pyrite
weathering processes, which can be found especially in black clay shales.
Degradation of concrete drill cores relates also to growth pressure of
rhombohedral calcite crystals in concrete matrix (Hartshorn, Sharp, Swamy [3]).
-
Thaumasite
formation in experimental conditions studied (Crammond, Halliwell [2]). They
verified thaumasite formation of neutral sulphates ions added to concrete or by
sulphur acid action on concrete Oberholster, van Aardt, Brandt
[7]).
-
It can not be
excluded also affects of surrounding environment and the bedrock. The rocks
containing sulphides and organic matters are altered by acid solutions by the
formation of more stabile mineral forms. The typical example is pyrite decaying
with the formation of limonite and sulphur acid. It can be demonstrated (
-
ASR reaction
(in a limited degree – it was verified in 3 samples)
During the study of concrete samples was found by
optical microscopy that even highly degraded concrete contents anhydrated
clinker minerals. C3S and C2S are well distinguishable in
some cases. Tetracalciumalumoferrite is the most marked in case of
It was verified that in studied samples thaumasite
often with ettringite were formed gradually of ASR gels. Their occurrence was
confirmed by microanalyses. It can be formed hypothesis that important factor
of rupture deformation of concrete matrix with formation of fine fissures are
not only ASR gels but also following reactions induced by sulphur and
hydrocarbon acids, hydroxides of Al and Ca together with ASR gels with the
formation of especially thaumasite and in case of over-abundance of Ca2+
+ Al3+ also ettringite. Gypsum needle-like crystals are formed in
case of Ca ions presence (after formation of ettringite) together with low
concentrated sulphur acid. Calcite is formed as a latest mineral in case of
presence of remains of Ca hydroxide (Photo 1-10).
It can be understood that ettringite is usual product
of hydration and occurs both in fresh and degraded concretes. Ettringite causes
concrete decaying only in case of its excessive formation, which increases with
increasing age of concrete. Ettringite can not crystallize in free voids of
concrete microstructure. Ettringite occurrence, lower than critical can only
signalize alteration of microstructure (together with alteration of concrete
properties) but need not lead to concrete decay.
Acknowledgements
The research was supported by
References
1. Arya C,
Buenfeld NR, Newman JB. Factors influencing chloride-bearing
in concrete. Cem. Concr.
Res. 20: 1990. pp. 291-300
2. Crammond NJ, Halliwell M. The
thaumasite form of sulfate attack in concretes containing a source of carbonate
ions - A micro structural overview, in:
V. M. Malhotra (Ed), Proceedings 2nd CANMET/ACI Symposium on Advances in Concrete, ACI SP 154, 1995, pp.
357-380.
3. Hartshorn
SA, Sharp JH, Swamy RN. Thaumasite formation in portland-limestone cement
pastes. Cem Concr Res. 29 (199), 1993, pp. 1331-1340.
4.
Hawkins AB, Pinches GM. Sulfate analysis on black mudstones. Geotechnique.
37, 1987, pp.191-196.
5. Hobbs DW,
Taylor MG. Nature of the thaumasite sulfate attack mechanism in field concrete, 2000, Elsevier Science.
(Reprinted with permission from Cement and Concrete Research, Vol.30, No.4)
6. Sandover BR,
7. Oberholster RE,
van Aardt JHP, Brandt MP. Durability of
cementitious systems, in: P. Barnes (Ed), Structure and performance of cements,
|
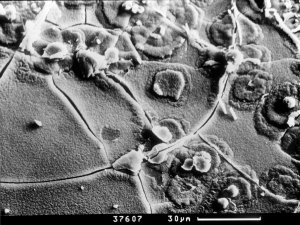
Photo 1. Formation of radial-like
forms of portlandite of gel. SEM - |
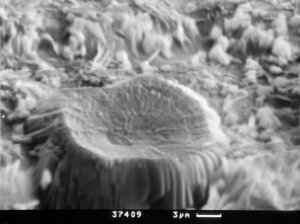
Photo 2. Detail view – one of
formatting forms. SEM - |
|
Photo 3. Formation of first thaumasite
and ettringite crystals. SEM - |
Photo 4. Advanced state of
thaumasite formation. SEM - |
|
Photo 5. Needle-like forms of
thaumasite beside decaying gel. SEM - |
Photo 6. Ettringite, thaumasite,
gel, portlandite. SEM - |
|
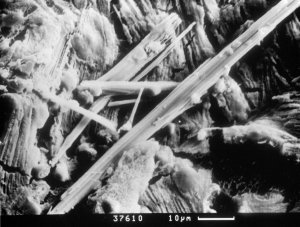
Photo 7. Clumps of ettringite
needles. SEM - |
Photo 8. Columnar shape forms of
thaumasite. SEM - |
|
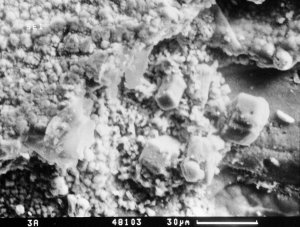
Photo 9. Rhombohedral calcite
crystals on thaumasite in highway concrete. SEM - |
Photo 10. Rhombohedral calcite
crystals beside thaumasite on the surface of interlocking concrete pavement.
SEM - |